
It is not uncommon for small biotech and biopharma startup companies to overlook Quality & Compliance when formulating their drug development plans and budgets. However, industry guidance from the International Conference on Harmonization (ICH) recommends that serious consideration be given to Quality Assurance (QA) very early in the process.
The diagram below highlights the major interdisciplinary activities that need to be engaged when advancing drug candidates from Late Discovery to IND/CTA submission and clinical trial initiation. Smaller companies routinely engage 3rd party service providers to handle some or all these functions, and it is often incorrectly assumed that as long as the contracted deliverables are received, everything will be in place to enter into clinical trials.
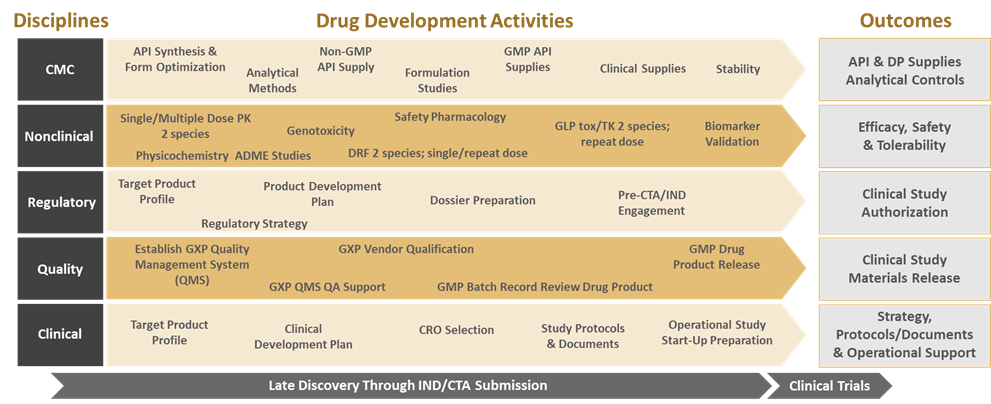
A robust Pharmaceutical Quality System (PQS), as described in ICH Q10, is critical to the development and manufacture of pharmaceutical drug substances and drug products throughout the product lifecycle, including biotechnology and biological products. The scope of this guidance includes all outsourced activities and purchased materials. For this reason, it is important that Quality Assurance is a part of the planning conversation in the early stages of development. Companies who do not pay proper attention to Quality run the risk of downstream consequences such as delayed study starts and product approvals due to regulatory agency actions (e.g., clinical study holds, FDA 483s, Warning Letters).
To illustrate this point, an FDA Warning Letter was recently issued to a small company regarding their use of contracted services. One of the statements from the Warning Letter clearly delineates the regulatory expectations related to management of outsourced activities: “Your firm lacks appropriate oversight and procedures to manage contract manufacturing organizations (CMOs) used on behalf of your firm for the manufacturing, packaging, and distribution of your drug products. You are ultimately responsible for CGMP activities you perform.” The Warning Letter concludes with two additional points to consider regarding the use of contracted services:
- “Drugs must be manufactured in conformance with CGMP. FDA is aware that many drug manufacturers use independent contractors such as production facilities, testing laboratories, packagers, and labelers. FDA regards contractors as extensions of the manufacturer.”
- “You are responsible for the quality of your drugs regardless of agreements in place with your contract facilities.”
For more information about FDA’s expectations for use of contracted services, see FDA’s guidance document Contract Manufacturing Arrangements for Drugs: Quality Agreements at https://www.fda.gov/media/86193/download.
Ronay LeBlanc is Vice President of Quality & Compliance at NGT BioPharma Consultants.
